Dimethylglyoxime is a chemical compound described by the formula CH 3 C (NOH)C (NOH)CH 3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the di oxime derivative of the diketone butane-2,3-dione (also known as diacetyl). The spectro photometric determination of nickel with dimethyl glyoxime (DMG) is widely used, and the reaction is carried out in aqueous alkaline medium. 21–23The procedure involves oxidation of Ni2+ by bromine, iodine or persulfate.
Dimethylglyoxime (dmg) is a bidentate ligand chelating large amounts of metals. Only two dmg molecules per metal center are needed, because Ni (dmg)22 + has a square-planar geometry. To the aqueous array, add 1 per cent dmg. What is the role of DMG in analytical chemistry? 1,2-DIIMINE LIGANDS (CHELATING AGENTS) An example of a 1,2-diimine is the dimethylglyoximato ligand (which somewhat resembles a conjugated diene for Diels-Alder reactions). Name: bis-(DMG)nickel(II) As a general rule, 'bis' is used to indicate two of the same, complicated ligand. Chemical formula: 'Ni'('DMG')2 If we call one of the imine carbons 'carbon-1', then the other imine carbon is one. Dimethylogloxine (SMH) is a bidentate Ligand that chelates a large no. Only two dmg molecules are required per metal center because Ni (dmg)2^2 + has a square - planer geometry. Hope it helps you plz mark me as brainliest.
monodentate ligands
Burn dmg to micro sd card bash ubuntufocusnew. Denticity refers to the number of donor groups in a single ligand that bind to a central atom in a coordination complex.[1][2] In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The word denticity is derived from dentis, the Latin word for tooth. The ligand is thought of as biting the metal at one or more linkage points. The denticity of a ligand is described with the Greek letter κ ('kappa').[3] For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
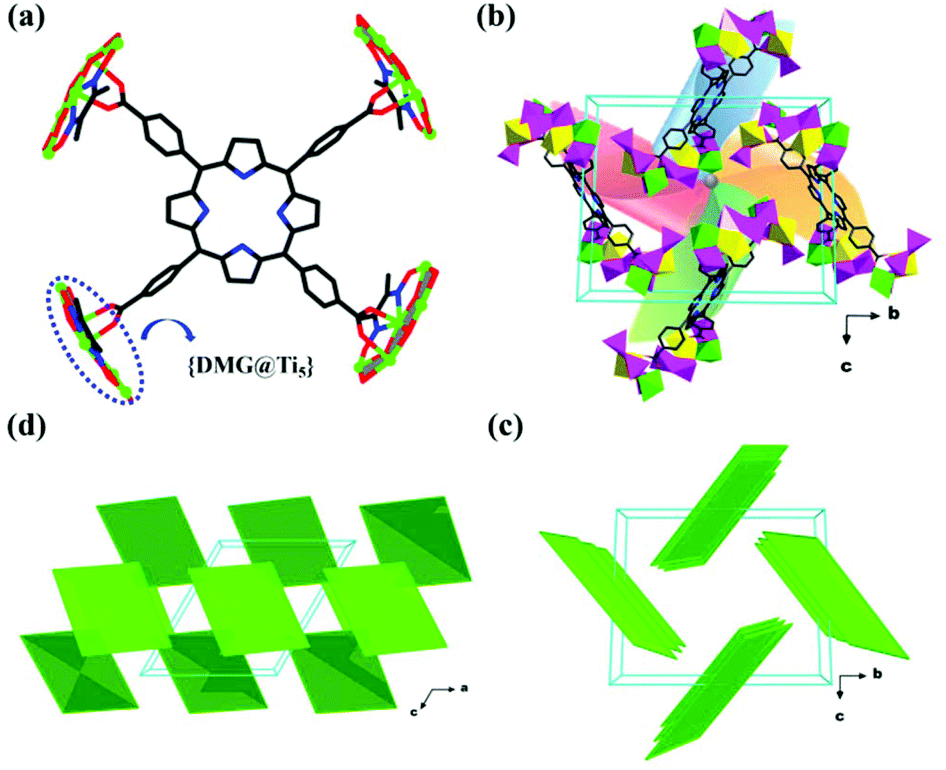
Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used.[4]Bridging ligands use the μ ('mu') notation.[5][6]
Classes[edit]
Polydentate ligands are chelating agents[7] and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more binding sites of the ligand are unused. Such sites can be used to form a bond with another chemical species.
- Bidentate (also called didentate) ligands bind with two atoms, an example being ethylenediamine.
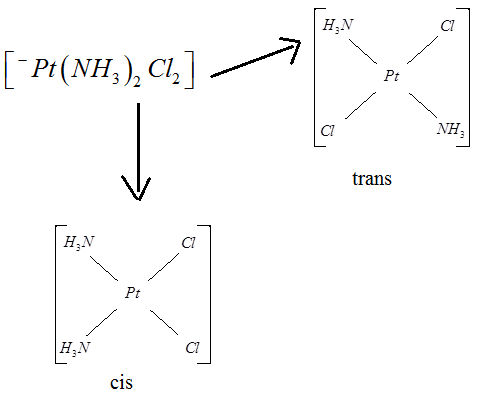
- Tridentate ligands bind with three atoms, an example being terpyridine. Tridentate ligands usually bind via two kinds of connectivity, called 'mer' and 'fac.' 'fac' stands for facial, the donor atoms are arranged on a triangle around one face of the octahedron. 'mer' stands for meridian, where the donor atoms are stretched out around one half of the octahedron. Cyclic tridentate ligands such as TACN and 9-ane-S3 bind in a facial manner.
- Tetradentate ligands bind with four donor atoms, an example being triethylenetetramine (abbreviated trien). For different central metal geometries there can be different numbers of isomers depending on the ligand's topology and the geometry of the metal center. For octahedral metals, the linear tetradentate trien can bind via three geometries. Tripodal tetradentate ligands, e.g. tris(2-aminoethyl)amine, are more constrained, and on octahedra leave two cis sites (adjacent to each other). Many naturally occurring macrocyclic ligands are tetradentative, an example being the porphyrin in heme. On an octahedral metal these leave two vacant sites opposite each other.
- Pentadentate ligands bind with five atoms, an example being ethylenediaminetriacetic acid.
- Hexadentate ligands bind with six atoms, an example being EDTA (although it can bind in a tetradentate manner).
- Ligands of denticity greater than 6 are well known. The ligands 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA) and diethylene triamine pentaacetate (DTPA) are octadentate. They are particularly useful for binding lanthanide ions, which typically have coordination numbers greater than 6.
Stability constants[edit]
In general, the stability of a metal complex correlates with the denticity of the ligands, which can be attributed to the chelate effect. Polydentate ligands such as hexa- or octadentate ligands tend to bind metal ions more strongly than ligands of lower denticity, primarily due to entropic factors. Stability constants are a quantitative measure to assess the thermodynamic stability of coordination complexes.
See also[edit]
External links[edit]
- EDTA chelation lecture notes. 2.4MB PDF - Slide 3 on denticity
References[edit]
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'denticity'. doi:10.1351/goldbook.D01594
- ^von Zelewsky, A. 'Stereochemistry of Coordination Compounds' John Wiley: Chichester, 1995. ISBN047195599X.
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'κ (kappa) in inorganic nomenclature'. doi:10.1351/goldbook.K03366
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'η (eta or hapto) in inorganic nomenclature'. doi:10.1351/goldbook.H01881
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'bridging ligand'. doi:10.1351/goldbook.B00741
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'µ- (mu) in inorganic nomenclature'. doi:10.1351/goldbook.M03659
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'chelation'. doi:10.1351/goldbook.C01012
Explain the difference between 1,2- and 1,3- chelating amines? Show example.
1 Answer
1,2-DIIMINE LIGANDS (CHELATING AGENTS)

An example of a 1,2-diimine is the dimethylglyoximato ligand (which somewhat resembles a conjugated diene for Diels-Alder reactions).
Name:
bis-(DMG)nickel(II)
Dmg Ligand Name
As a general rule, 'bis' is used to indicate two of the same, complicated ligand.
Chemical formula:
#'Ni'('DMG')_2#
If we call one of the imine carbons 'carbon-1', then the other imine carbon is one atom away (meaning, on 'carbon-2'). Hence, we can call this a 1,2-diimine.
Each dimethylglyoximato ligand (abbreviated DMG in compound names) has a net charge of #mathbf(-1)#, and each nitrogen acts as a binding site due to their lone pairs of electrons.
If you recall the chelate effect, it essentially states that the binding of more than one 'tooth' is entropically favored, relative to binding with one 'tooth'.
This two-'tooth' binding mode requires that the ligand's binding sites be cis (same-side). Therefore, DMG is a bidentate ligand, binding preferentially cis.
1,3-DIIMINE LIGANDS (CHELATING AGENTS)
An example of this that I can find in my book is the N,N'-diphenyl-2,4-pentanediiminato ligand.
Name:
Dmg Ligand Oxidation State

Dimethylglyoxime is a chemical compound described by the formula CH 3 C (NOH)C (NOH)CH 3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the di oxime derivative of the diketone butane-2,3-dione (also known as diacetyl). The spectro photometric determination of nickel with dimethyl glyoxime (DMG) is widely used, and the reaction is carried out in aqueous alkaline medium. 21–23The procedure involves oxidation of Ni2+ by bromine, iodine or persulfate.
Dimethylglyoxime (dmg) is a bidentate ligand chelating large amounts of metals. Only two dmg molecules per metal center are needed, because Ni (dmg)22 + has a square-planar geometry. To the aqueous array, add 1 per cent dmg. What is the role of DMG in analytical chemistry? 1,2-DIIMINE LIGANDS (CHELATING AGENTS) An example of a 1,2-diimine is the dimethylglyoximato ligand (which somewhat resembles a conjugated diene for Diels-Alder reactions). Name: bis-(DMG)nickel(II) As a general rule, 'bis' is used to indicate two of the same, complicated ligand. Chemical formula: 'Ni'('DMG')2 If we call one of the imine carbons 'carbon-1', then the other imine carbon is one. Dimethylogloxine (SMH) is a bidentate Ligand that chelates a large no. Only two dmg molecules are required per metal center because Ni (dmg)2^2 + has a square - planer geometry. Hope it helps you plz mark me as brainliest.
monodentate ligands
Burn dmg to micro sd card bash ubuntufocusnew. Denticity refers to the number of donor groups in a single ligand that bind to a central atom in a coordination complex.[1][2] In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The word denticity is derived from dentis, the Latin word for tooth. The ligand is thought of as biting the metal at one or more linkage points. The denticity of a ligand is described with the Greek letter κ ('kappa').[3] For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used.[4]Bridging ligands use the μ ('mu') notation.[5][6]
Classes[edit]
Polydentate ligands are chelating agents[7] and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more binding sites of the ligand are unused. Such sites can be used to form a bond with another chemical species.
- Bidentate (also called didentate) ligands bind with two atoms, an example being ethylenediamine.
- Tridentate ligands bind with three atoms, an example being terpyridine. Tridentate ligands usually bind via two kinds of connectivity, called 'mer' and 'fac.' 'fac' stands for facial, the donor atoms are arranged on a triangle around one face of the octahedron. 'mer' stands for meridian, where the donor atoms are stretched out around one half of the octahedron. Cyclic tridentate ligands such as TACN and 9-ane-S3 bind in a facial manner.
- Tetradentate ligands bind with four donor atoms, an example being triethylenetetramine (abbreviated trien). For different central metal geometries there can be different numbers of isomers depending on the ligand's topology and the geometry of the metal center. For octahedral metals, the linear tetradentate trien can bind via three geometries. Tripodal tetradentate ligands, e.g. tris(2-aminoethyl)amine, are more constrained, and on octahedra leave two cis sites (adjacent to each other). Many naturally occurring macrocyclic ligands are tetradentative, an example being the porphyrin in heme. On an octahedral metal these leave two vacant sites opposite each other.
- Pentadentate ligands bind with five atoms, an example being ethylenediaminetriacetic acid.
- Hexadentate ligands bind with six atoms, an example being EDTA (although it can bind in a tetradentate manner).
- Ligands of denticity greater than 6 are well known. The ligands 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA) and diethylene triamine pentaacetate (DTPA) are octadentate. They are particularly useful for binding lanthanide ions, which typically have coordination numbers greater than 6.
Stability constants[edit]
In general, the stability of a metal complex correlates with the denticity of the ligands, which can be attributed to the chelate effect. Polydentate ligands such as hexa- or octadentate ligands tend to bind metal ions more strongly than ligands of lower denticity, primarily due to entropic factors. Stability constants are a quantitative measure to assess the thermodynamic stability of coordination complexes.
See also[edit]
External links[edit]
- EDTA chelation lecture notes. 2.4MB PDF - Slide 3 on denticity
References[edit]
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'denticity'. doi:10.1351/goldbook.D01594
- ^von Zelewsky, A. 'Stereochemistry of Coordination Compounds' John Wiley: Chichester, 1995. ISBN047195599X.
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'κ (kappa) in inorganic nomenclature'. doi:10.1351/goldbook.K03366
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'η (eta or hapto) in inorganic nomenclature'. doi:10.1351/goldbook.H01881
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'bridging ligand'. doi:10.1351/goldbook.B00741
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'µ- (mu) in inorganic nomenclature'. doi:10.1351/goldbook.M03659
- ^IUPAC, Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book') (1997). Online corrected version: (2006–) 'chelation'. doi:10.1351/goldbook.C01012
Explain the difference between 1,2- and 1,3- chelating amines? Show example.
1 Answer
1,2-DIIMINE LIGANDS (CHELATING AGENTS)
An example of a 1,2-diimine is the dimethylglyoximato ligand (which somewhat resembles a conjugated diene for Diels-Alder reactions).
Name:
bis-(DMG)nickel(II)
Dmg Ligand Name
As a general rule, 'bis' is used to indicate two of the same, complicated ligand.
Chemical formula:
#'Ni'('DMG')_2#
If we call one of the imine carbons 'carbon-1', then the other imine carbon is one atom away (meaning, on 'carbon-2'). Hence, we can call this a 1,2-diimine.
Each dimethylglyoximato ligand (abbreviated DMG in compound names) has a net charge of #mathbf(-1)#, and each nitrogen acts as a binding site due to their lone pairs of electrons.
If you recall the chelate effect, it essentially states that the binding of more than one 'tooth' is entropically favored, relative to binding with one 'tooth'.
This two-'tooth' binding mode requires that the ligand's binding sites be cis (same-side). Therefore, DMG is a bidentate ligand, binding preferentially cis.
1,3-DIIMINE LIGANDS (CHELATING AGENTS)
An example of this that I can find in my book is the N,N'-diphenyl-2,4-pentanediiminato ligand.
Name:
Dmg Ligand Oxidation State
dichloro(nacnac)nickel(II)
As a general rule, complicated ligands tend to be in parentheses.
Chemical formula:
#['Ni'('nacnac')'Cl'_2]^(-)#
Fortunately, this ligand has a simple 'nickname': nacnac.
Like DMG, nacnac is a bidentate ligand, and its two binding sites are the two nitrogens, each using their lone pair of electrons. However, its imine carbons are 1,3 to each other instead.
This ligand also has a net charge of #mathbf(-1)#.
It substantially favors binding cis to the metal, again due to the chelate effect favoring the binding of both 'teeth' rather than only one of them.
